Fuel, Oil and Coolant Specifications
Depletion of all types of inhibitors occurs through normal operation. Therefore, strength levels must be maintained by
the addition of inhibitors at prescribed intervals. Always follow the supplier's recommendations on inhibitor usage and
handling.
Chromates
Sodium chromate and potassium dichromate are two of the best and most commonly used water system corrosion
inhibitors. However, the restrictive use of these materials, due to ecology considerations, has de-emphasized their use in
favor of non-chromates. Care should be exercised in handling these materials due to their toxic nature.
Chromate inhibitors should not be used in permanent type antifreeze solutions. Chromium hydroxide, commonly called
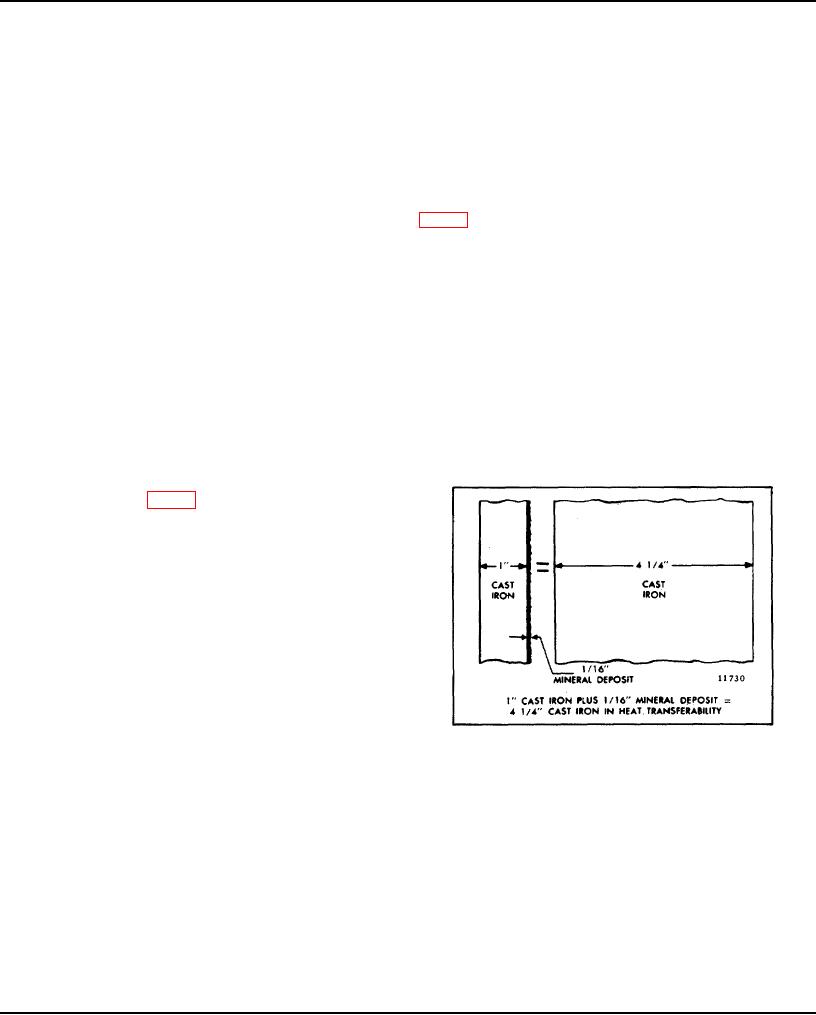
"green slime", can result from the use of chromate inhibitors with permanent type antifreeze. This material deposits on
the cooling system passages, reducing the heat transfer rate (Fig. 2) and results in engine overheating. Engines which
have operated with a chromate-inhibited water must be chemically cleaned before the addition of permanent antifreeze.
A commercial heavy-duty de-scaler should be used in accordance with the manufacturer's recommendation for this
purpose.
Soluble Oil
Soluble oil has been used as a corrosion inhibitor for many years. It has, however, required very close attention relative
to the concentration level due to adverse effects on heat transfer if the concentration exceeds 1% by volume. For
example: 1 1/4% of soluble oil in the cooling system increases fire deck temperature 6% and a 2 1/2% concentration
raises fire deck temperature up to 15%. Soluble oil is not recommended as a corrosion inhibitor.
Non-chromates
Non-chromate inhibitors (borates, nitrates, nitrites, etc.) provide corrosion protection in the cooling system with the basic
advantage that they can be used with either water or a water and permanent antifreeze solution.
INHIBITOR SYSTEMS
An inhibitor system (Fig. 3) is a combination of chemical
compounds which provide corrosion protection, pH control
and water softening ability.
Corrosion protection is
discussed under the heading Corrosion Inhibitors. The pH
control is used to maintain an acid-free solution. The water
softening ability deters formation of mineral deposits.
Inhibitor systems are available in various forms such as
coolant filter elements, liquid and dry bulk inhibitor
additives, and as an integral part of permanent antifreeze.
Fig. 2 - Heat Transfer Capacity
Coolant Filter Elements
Replaceable elements are available with various chemical inhibitor systems. Compatibility of the element with other
ingredients of the coolant solution cannot always be taken for granted.
Problems have developed from the use of the magnesium lower support plate used by some manufacturers in their
coolant filters. The magnesium plate will be attacked by solutions which will not be detrimental to other metals in the
cooling system. The dissolved magnesium will be deposited in the hottest zones of the engine where heat transfer is
most critical. The use of an aluminum or zinc support plate in preference to magnesium is recommended to eliminate
the potential of this type of deposit. High chloride coolants will have a detrimental effect on the water softening
capabilities of systems using ion-exchange resins. Accumulations of calcium and magnesium ions removed from the
coolant and held captive by the zeolite resin can be released into the coolant by a regenerative process caused by high
chloride content solutions.
Page 72

